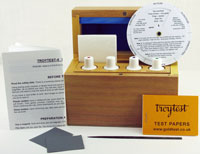
Guide to chemical testers (gold-testing acids)
ACID TEST KITS, WHICH ONE TO BUY
(various models of Quicktest or Troytest tester and what the various bottles test for)
TO BUY: CLICK ON THE PICTURE

TO WATCH A DEMONSTRATION, CLICK ON THE PICTURE
CONTENTS
EIGHT QUESTIONS ANSWERED
WHAT IS THE DIFFERENCE BETWEEN THE MODELS
WHAT IS EACH BOTTLE USED FOR?
LIMITATIONS
SAFETY INFORMATION
HOW TO USE THE BOTTLES and HOW TO DISPOSE OF OLD ACID
JUST HOW DANGEROUS ARE THE ACIDS?
COSHH DATA
SUMMARY OF SAFETY INFORMATION
This article is about testing with Troytest and Quicktest acid sets, the method, and the difference between the different sets.
If you are not ready for this and just want to know if acid-testing is right for you, see 12 methods of testing gold, silver and platinum.
Terminology: throughout this article the term METAL is used in its chemical sense, not in the colloquial sense (still used by some jewellers) of anything that is not gold or silver.
EIGHT QUESTIONS ANSWERED
-
How reliable is the acid test?
A. Very reliable. It has even entered the English language, we say (referring to ultimate proof), "The acid test is…"
-
Will the acid test 'test' through gold/silver plating?
A. No, you must file the item first (a very fine needle-file is included with each testing set) so that you are testing whatever might be underneath any plating. You must also file the item before carrying out a test an electronic tester (any electronic tester) unless the plating is very thin.
-
Do I put the acid on the filings that have been taken off with the needle file?
A. No, you put the acid on the actual item
-
Do acids cause any damage?
A. You must file it in a place where it won't show. If you can't file it you can't test it. On 9ct (and sometimes 14ct) the acid it leaves a stain. This can be polished off with a cloth or polishing sticks.
-
Are acids easy to use?
A. You need to spend a few minutes practicing, but the instructions are very clear, it really is not difficult.
-
Do I need to wear special protective clothing when using acids?
A. As with all chemicals (e.g. household cleaners) you may wish to wear an apron to protect clothes. Small drops of acid stain small spots of skin yellow (if you’re careful, you shouldn't spill any!) and it can take a few days for the skin to grow back. I recommend special acid-proof gloves. If using glass bottles (only 1% of our customers buy these, most use plastic bottles) wear goggles to protect against accidental splashes. You can read about other safety equipment and what we do, and don't, recommend.
-
I am colour-blind, how will I see the colour-change of the acid?
A. Unlike other brands you don't have to distinguish between reds, greens and browns. There are special instructions explaining what the "colour change" looks like to anyone who is red-green colour blind.
-
Can acids test for white metals including testing platinum?
A. Yes, the Troytest (4-bottle set) and Troytest-5 (5-bottle set) can distinguish white gold, platinum, steel and Palladium.
There is a huge amount of information in The Gold & Silver Buyer's Handbook.
WHAT IS THE DIFFERENCE BETWEEN THE MODELS
All sets are supplied in a strong, lockable, wooden box with the bottles and accessories. The wooden box is packed in a sturdy cardboard outer box. To buy these, click here.
If you buy loose bottles (not a complete set) you are buying a 'refills'. These do not include instructions or accessories, they are replacement bottles for your boxed set. Do not order loose bottles unless you know how to use them and you have a secure (preferably lockable) box. To buy refill bottles click here.
Quicktest 3
The Quicktest 3 tests for all carats of gold and also silver
This is the 'standard' set for testing gold and silver
Accessories: steel file, magnet, instruction manual (and summary of instructions fixed to lid)
Troytest 4
The Troytest 4-Bottle set is the same as the Quicktest-3, but with a fourth bottle for distinguishing 18ct white, steel, platinum and Palladium. But this (fourth) bottle has to be used in conjunction with 14-24ct bottle, so it can't be used on its own.
Accessories: instruction manual, indicator disc, polishing paper, magnet, sample of copper
Up until 2008 this set was named the TROYTEST MK V.
Troytest 5
The Troytest 5-Bottle set is the same as the 4-bottle set, but with an additional bottle for 8ct (quite rare) and also an unusual 9ct mixture (alloy) that contains a lot of zinc and can be confused with non-gold when using the first (9ct) acid.
Accessories: instruction manual, indicator disc, polishing paper, magnet, sample of copper
WHAT IS EACH BOTTLE USED FOR?
The Amber (Silver) Bottle
Appearance: the bottle is labelled AMBER FLUID (silver), the colour of the fluid is amber.
Purpose: to tell if a white metal is silver, typically .925, though there's a slight reaction on .800
The White (9ct) Bottle
Appearance: the bottle is labelled WHITE FLUID (9ct), the fluid is clear.
Purpose: to tell if a metal is not gold, or if it is 9ct, or if it is better than 9ct. If it is better than 9ct it won't tell you what it is, you must move on to the 14-24ct bottle.
The Blue (14-24ct ) Bottle
Appearance: the bottle is labelled BLUE FLUID (14-24ct), the fluid varies from light yellow to deep yellow.
Purpose: tests for 14 to 24ct.
Tip: this fluid is to test from 14 to 24ct having first used the 9ct fluid to test for 9ct / non-gold. It is also used in combination with the GREEN fluid to test for white gold, steel, platinum and Palladium.
The Green (platinum) Bottle
Appearance: the bottle is labelled GREEN FLUID, the fluid is clear.
Purpose: to distinguish 18ct WHITE gold from stainless steel, platinum and Palladium. This fluid must be used in conjunction with the BLUE fluid, it cannot be used on its own.
TIP: if the metal is magnetic or if, when you file the item, you can feel that the metal is hard (as hard as the steel file) - it cannot possibly be gold or silver or platinum, there is no need to use acid. (Though being magnetic does not mean that it's steel, some steel is not magnetic).
The Clear (high-zinc 9ct) Bottle
Appearance: the bottle is labelled CLEAR FLUID (8-9ct), the fluid is clear.
Purpose: to distinguish a particular 9ct alloy that contains a high level of zinc from 'standard' 9ct, and at the same time to give an indication of very low-grade gold such as 8ct.
Tip: this is not an easy fluid to use and should not be used in place of the standard 9ct bottle. Also, since the high-zinc alloy is quite recent, all items should be hallmarked, so test the hallmark link, check that the remainder of the item has the same reaction, if in doubt check against a piece of copper, only if there is still doubt need you use this bottle.
Plastic bottles are supplied as standard when you order a testing kit.
Plastic bottles
Advantages: if knocked over it won't spill more than one drop; childproof cap; no extra 'bits' to think about, just the bottle with its integral dropper.
Disadvantage: the dropper dispenses drops of a fixed size, you cannot apply smaller drops.
Glass bottles
Advantages: you can use the the applicator to apply tiny drops of acid, easy to control, doesn't drip.
Disadvantages: if you knock it over you spill acid everywhere, very dangerous; if dropped it could smash; when you take the lid off you get a strong whiff of fumes. In theory (we haven't tried this) if it gets hot enough, e.g. in a car in the sun on a hot day, it will explode.
HINTS AND TIPS
When first learning to use the acids, check the reaction times against pieces of gold of known purity, any hallmarked items will suffice. This should also be done every few weeks to check the strength of the acids (a sample of copper is included with each set to check the "non-gold" reaction).
Always file the item with a steel file (you apply the acid to the filed area of the item) - otherwise if the item is plated, you will merely test the plating. Even if you are certain it isn't plated, clean the area to be tested - this is because you are observing a colour-change in the acid, and this is difficult to see this against a background of dirt.
To test high carat purity (22ct to 24ct) very accurately use a scrap British gold coin (which is exactly 916 parts per thousand): place acid on both coin and unknown gold and compare reaction times (i.e. - is the reaction on the test-item very slightly slower or very slightly faster than on the coin?)
To see what is meant by the 9ct acid 'fizzing spectacularly on non-gold', try it on a piece of copper, this is quite unlike the reaction when it turns dark then green-ish, then slowly bubbles on 9ct (especially when the acid is new).
Always start with the 9ct bottle, this is most important. This is because the 14-24ct bottle will give similar results on 9ct as it does on 14ct.
...however...
if the metal is white you may wish to start with the SILVER bottle instead. Usually you will know whether to test for gold or for silver, a candlestick or tray will not be gold or platinum, an intricately-made antique-looking ring with a probable diamond is not likely to be silver. But if there is doubt, start with the SILVER bottle when testing white metal.
Gold of 22ct to 24ct is always yellow, lower-carat gold can be yellow, red, pink or white; silver and platinum are always white.
Gold, silver and platinum are not magnetic. A magnet is not, however, a test for steel since not all steel is magnetic.
Gold, silver and platinum are soft compared with steel, you will notice the moment you try to file it. Similarly, lead is so soft that it instantly clogs the file (and can be cut with a penknife). Gold, silver and platinum are heavy (as is lead), aluminium is noticeably lightweight.
Another method of testing with acid uses a streak board (touchstone). This is a piece of black stone or fine slate across which the gold is drawn so that it leaves behind a gold coloured streak. The test-item and two or more items of known purity are each streaked horizontally across the board. The acid is then placed vertically across all the streaks at once. The rate at which the streaks dissolve is then observed - details.
The hints and tips, above, give you a flavour of The Gold & Silver Buyer's Handbook (184 pages).
LIMITATIONS
Foolish is the gold buyer who spends large amounts of money then tests the items having not studied the instructions. Sit down at a firm table with a good light and a selection of gold items and test two or three items of each carat so that you get used to the readings. Before you open a bottle have a tissue ready to catch any drips.
Your hand must be steady enough to place a drop of acid on a tiny area you have filed, and your eyesight must be good enough to see the reaction. If this is not possible there is no way you will be able to use acid. So if you need reading glasses - find them before you start; if you need an eyeglass to see the reaction, get one before you start.
Acid is not suitable for testing gold dust (the dust soaks the acid up before the reaction can be noted) or for testing gold melted by amateurs (it can emerge very porous and will not be homogenous).
Rock containing gold is also problematic, if it's alluvial and you can actually see nuggets of gold sticking out, hit them with a hammer and hope they come off cleanly. If you have flecks of gold in the rock the only way to get the gold out would be to crush the rock, dissolve the gold out mercury or cyanide, then reclaim the gold - in other words, forget it.
SAFETY INFORMATION
All the fluids are toxic and corrosive and should be treated with extreme care
Do not breathe fumes
Avoid skin contact. In the event of skin contact, wash immediately with plenty of water
In case of eye contact, wash with plenty of water and seek medical advice
Keep out of reach of children
In case of spillage, flood with plenty of water
Emergency? Go to COSHH data
HOW TO USE THE BOTTLES and HOW TO DISPOSE OF OLD ACID
Ensure you have a tissue ready before you start, you will need to mop up spots of acid. A free tissue and leaflet is provided with each refill bottle and with each set.
Remove shrink seal and tape. The cap is tamper-proof, a plastic ring which will break the first time the cap is removed. To open, push down and twist to the left to release, then gently unscrew. This is the same as childproof caps on medicine bottles.
When opening the bottle carefully mop up, with the tissue, any acid on the outside of the nozzle.
To apply, turn the bottle upside down, gently squeeze and watch the acid slowly move down the nozzle. If you can't see this, stop, find your reading glasses or a magnifier, start again. Let a blob of acid form on the outside of the nozzle and touch it on the metal. Do not squeeze so hard that you squirt acid! Have a tissue ready to catch any drops that spill, wear acid-proof gloves.
After each use, mop up any spots of acid from the outside of the nozzle (the 18ct / Blue Fluid always gets onto the outside of the nozzle). Have the tissue ready.
When replacing the cap, press it downwards as you tighten it (you will feel it getting tighter) otherwise it will leak.
Each fluid will last between one and two years, the date of manufacture is printed on the bottle label.
If you are using the glass bottles and you want to transfer the acid, do this in a sink so that you can turn the tap on and wash any spills. Keep your face well away from any fumes that come out of the bottles, and take great care not to splash. Wear acid-proof gloves and goggles.
If you have old PLASTIC acid bottles, destroy any remaining acid as follows:
Go to a sink, turn on the taps. Gently squeeze out any remaining acid, then rinse it out like this:
Squeeze the empty bottle, tip the end into the flowing water, let go, clean water will be sucked into the bottle, squeeze it out into the flowing water. Do this or three or four times to ensure no acid remains. Now gently wash out the lid (taking care not to splash). The bottle is now clean and can be thrown away.
If you have old GLASS acid bottles, destroy any remaining acid as follows:
Go to a sink, turn the taps on, gently pour any remaining acid into the flowing water, gently wash out the bottle, gently wash out the cap, take care not to splash. All this is safe, this how acid is destroyed in a laboratory.
JUST HOW DANGEROUS ARE THE ACIDS?
Here are four short 'case histories' which will, we hope, put the danger in perspective.
The Case of the Stained Hand
A lady called to say she had spilt some acid on her hand a few days previously. She said, "It's stained my hand yellow and I've tried everything to wash it off and my hand is still yellow, what should I use to clean it?" She was horrified to learn that the 'yellow stain' was, in fact, a chemical burn and was not going to 'wash off'. But she was relieved to hear that the skin would grow back over the next few weeks.
Conclusion: If you do have an accident with the acid, don't panic, keep calm, wash the acid off under the tap. Many jewellers continually spill tiny drops on their hands and their fingers are always stained yellow. If you are careful there is no reason to ever spill drops of acid. If you manage to squirt acid over your skin and do not wash it (under a tap for about five minutes) you may wish to seek medical advice.
The Case of the Child
A distraught father telephoned. He had been using the acid and, against all the warnings, had had left the cap off, had left it within reach of a three-year-old, and had then left the room. The child spilt the acid down her leg, the parents did not follow the safety precautions, did not wash the acid off, and by the time the child arrived at hospital the acid had burnt down to the bone, the child needed major surgery and will be scarred for life.
Conclusion: Treat the acid as you would any other household chemical (bleach, ammonia etc). KEEP IT AWAY FROM CHILDREN and if there is an accident, follow the safety precautions.
The Case of the Sudden Illness
A man telephoned to say that he had used the acid, had accidentally sniffed some of the fumes, and a few hours later he felt sick and dizzy. He went to his G.P. who said that it was most unlikely that his symptoms had anything to do with the acid.
Conclusion: Regarding sniffing acid: it is not to be advised, and certainly not on a regular basis, it is not good for the lungs. However, don't panic if you accidentally sniff it just very occasionally, there really is no need to rush to the doctor. Jewellers who have various bottles, old and new, will often sort them by deliberately sniffing the fumes, if it makes them cough and splutter it's good - it's a fresh bottle...but this is not to be advised, for instance regular sniffing Blue Fluid can cause hydrochloric acid to accumulate in the lungs and over a long period that will cause medical problems. If you are storing dozens or hundreds of bottles (e.g. for distribution within a large company), keep them in a well-ventilated area well away from staff.
If you don't feel well you might be ill, quite irrespective of the acid, so please do whatever you usually do when you feel ill.
One word of caution: if you work with other chemicals that also produce fumes, especially if you don't have adequate ventilation, it is possible that the combination of fumes could make you ill.
The Case of the Eye
A customer telephoned to say that his friend might have got some acid in his eye. We asked when this happened and he said a few minutes ago; we asked where the friend was and he said, standing right here; we asked how sore the eye felt and he asked his friend and his friend said very sore; we gave the official advice which is to hold the eye open under a running tap for at least ten minutes; he asked if he should seek medical advice and (since he had asked) we said yes - we had to assume he had got acid in his eye, we couldn't possibly tell him, "It's probably nothing" when we had no way of knowing.
Conclusion: Please be aware of two extremes. If the person is screaming with pain as their eye dissolves into their brain, do not telephone us for advice, get that eye forced open under a running tap and dial 999. At the other extreme, if you think you may have had some acid on your finger and rubbed your eye but really don't know if you've rubbed acid or dirt (note how black your hands become from dirt when handle old jewellery) - keep calm, the eye 'feeling irritable' does not constitute a major injury, you will KNOW if you have acid in your eye!! - give it a wash and see how you feel in a few minutes.
Incidentally, if you wash yourself in icy cold water, the cold will make the skin go completely numb, so do not panic, the feeling will come back when the skin warms.
COSHH DATA
9ct Bottle ('white fluid') Nitric Acid above 50% EEC No. 231-714-2 CAS No. 7697-37-2
18ct Bottle ('blue fluid') Nitric Acid below 50% EEC No. 231-714-2 CAS No. 7697-37-2
Hydrochloric Acid above 50% EEC NO.231-595-7 CAS No. 7647-01-0
Silver Bottle ('amber fluid') Nitric Acid above 14.5% EEC No. 231-714-2
Chromium (VI) oxide below 12.2% EEC No. 215-607-8
HAZARDS IDENTIFICATION Toxic if swallowed. Causes severe burns.
FIRE FIGHTING Explosive with combustible material. May evolve toxic fumes in fire.
HANDLING AND STORAGE Store at room temperature (below 15 degrees Celsius recommended). Keep well closed and protected from direct sunlight and moisture. Store away from combustible materials.
SUMMARY OF SAFETY INFORMATION
All the fluids are toxic and corrosive and should be treated with extreme care
Do not breathe fumes
Avoid skin contact. In the event of skin contact, wash immediately with plenty of water
In case of eye contact, wash with plenty of water and seek medical advice
Keep out of reach of children
In case of spillage, flood with plenty of water
1. This advice is for clothing soaked in acid, does not apply to tiny drops of acid which will rot tiny amounts of fabric.
2. This advice is for serious burns, e.g. spilling an entire bottle of acid over the skin, most jewellers completely ignore small drops on the skin - though it is acid, it does burn, please give it a thorough wash under a tap.
3. This refers to drinking the acid. If you have a residue on your finger and touch your mouth, none of this applies.